This was corroborated by the National Lung Screening Trial,a during which annual lung screenings did not reduce the number of patients who were diagnosed with extensive-stage disease, nor did it have an impact on survival in SCLC.5,8,b
Symptomatic at Presentation
There is no effective screening test to detect SCLC early. By the time of diagnosis, patients present with multiple symptoms, consistent with the rapid progression of the disease.1-5
- Due to its rapid spread, ~70% of patients are diagnosed with extensive disease at the time of diagnosis, creating observable symptoms3,6,7
High Percentage of Extensive-Stage SCLC Detected by Low-Dose CT2


ES=extensive-stage; CT=computed tomography; OS=overall survival.
aStudy was conducted to determine whether annual screenings of low-dose CT scans could reduce mortality from lung cancer. 53,454 participants at high risk for lung cancer were enrolled from August 2002 through April 2004, screening took place from August 2002 through September 2007, and individuals were followed for events that occurred through December 31, 2009.8
bLung cancer was inclusive of bronchioloalveolar carcinoma, adenocarcinoma, squamous-cell carcinoma, large-cell carcinoma, non-small-cell carcinoma or other, small-cell carcinoma, carcinoid, and unknown.8
RAPID-ONSET SMALL CELL LUNG CANCER SYMPTOMS
- SCLC typically presents as a large hilar mass and bulky mediastinal lymphadenopathy and is characterized by rapid-onset symptoms that begin prior to initial diagnosis4,9,10
- Due to rapid tumor growth and widespread metastases, most patients with SCLC are symptomatic at
presentation. The duration of symptoms is typically less than 3 months3
- In a Surveillance, Epidemiological, and End Results (SEER) analysis of Medicare claims data, 75% of patients were hospitalized within the first 90 days following diagnosis11,*
- Signs and symptoms depend on the type of tumor growth and spread3
*5498 patients with ES-SCLC were identified as aged ≥66 years between January 1, 2007 and December 31, 2011. Patients were followed from diagnosis until death, coverage change, or December 31, 2013.11
Presentation and Select Symptomology, Diagnosis, and Metastases

PRESENTATION AND SELECT SYMPTOMOLOGY3,4,7,9,10,a
LIMITED-STAGE SCLC
- Respiratory conditions:
- Worsening cough, hemoptysis, wheezing, dyspnea
- Compression of the:
- Esophagus, with dysphagia
- Laryngeal nerve, left vocal cord paralysis
EXTENSIVE-STAGE SCLC
- In addition to the symptoms listed above, there are additional symptoms from distant metastases:
- Fatigue, anorexia
- Neurological compromise
- Bone pain from bone mets
- Weight loss and debility

DIAGNOSIS4,12,13
Continues with:
- CT imaging of the chest, abdomen, and pelvis
- Biopsy
- CT or MRI imaging of the brain

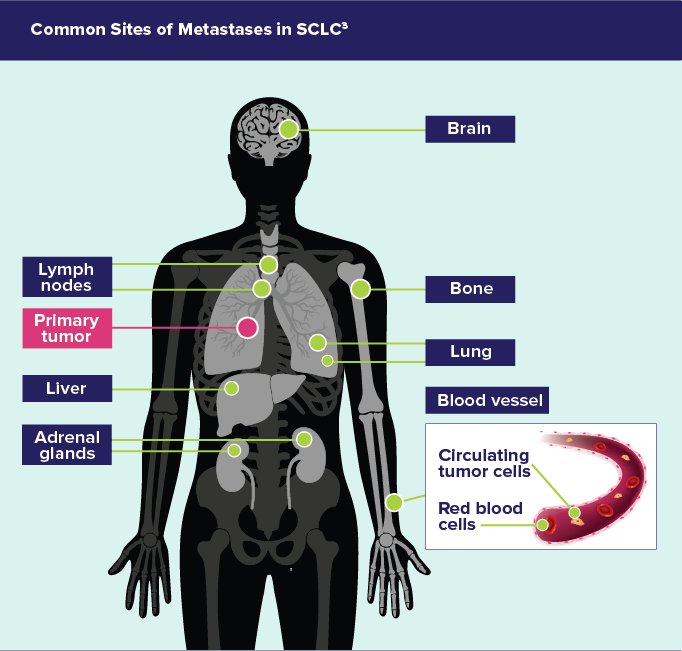
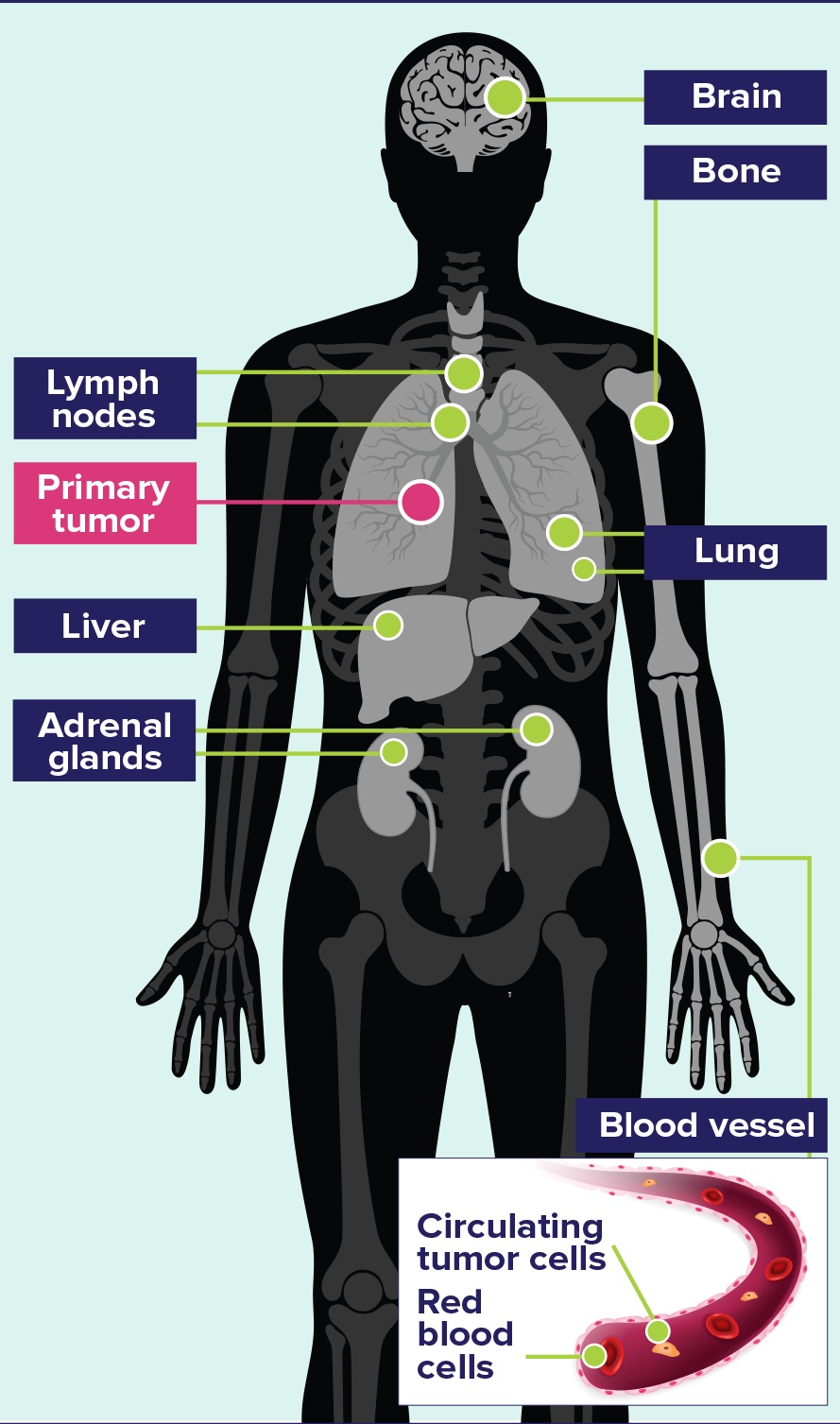
METASTASES3
- Mediastinal lymph nodes are common, and metastatic spread is often radiologically evident
- Distant metastatic spread commonly involves the lymph nodes and reaches the contralateral lung, brain, liver, adrenal glands, bone; and into blood vessels
MRI=magnetic resonance imaging.
aThis is not a comprehensive list of symptoms.
Common Sites of Metastases in SCLC3
RECOGNITION OF ONCOLOGIC EMERGENCIES CAN BE IMPERATIVE, AS DIAGNOSIS TYPICALLY OCCURS AFTER A RELATED CONDITION EMERGES14,a
Condition
Common Presenting Signs and Symptoms
Syndrome of
inappropriate antidiuretic hormone (SIADH)14
- Hyponatremia
- Nausea
- Vomiting
- Constipation
- Muscle weakness
Superior vena cava syndrome14
- Facial edema
- Cough
- Dyspnea at rest
- Hoarseness
- Chest and shoulder pain
- Collateral venous circulation (chest wall)
aOther oncologic emergencies that could be associated with SCLC include: tumor lysis syndrome, hypercalcemia of malignancy, febrile neutropenia, hyperviscosity syndrome, malignant epidural spinal cord compression, malignant pericardial effusions, and extravasation.14
PARANEOPLASTIC SYNDROMES CAN BE AN IMPORTANT DISTINCTION BETWEEN SCLC AND OTHER TUMORS
- Because SCLC is the most common solid tumor to cause paraneoplastic syndromes,10,15 understanding these syndromes provides opportunities for early intervention and management15
- Paraneoplastic syndromes are a collection of signs and symptoms associated with neoplastic disease, caused
by primary tumors from a distant site and does not involve the primary tumor15
- These syndromes can precede diagnosis or present with limited-stage SCLC, and can also occur at the time of recurrence or metastatic disease15
Common Paraneoplastic Syndromes
-
Caused by inappropriate production of ACTH and cortisol
Occurs in 1%-5% of patients
Symptoms include edema, muscle weakness, hypertension, weight gain, and metabolic alkalosis with hypokalemia
-
Caused by onconeural antibodies binding to calcium channels
Occurs in 1%-3% of patients
Symptoms include proximal muscle weakness
-
Caused by onconeural antibodies leading to site-specific inflammation and neuronal loss
Occurs in 1% of patients
Symptoms include cognitive dysfunction with severe memory impairment, seizures, and psychiatric features, including depression, anxiety, and hallucinations
ACTH=adrenocorticotropic hormone.
SCLC is a rapidly progressive and aggressive tumor.
As patients are typically symptomatic at
presentation, early recognition of symptoms and timely diagnosis are important.3,4,6
DIAGNOSING SCLC
- To distinguish SCLC from NSCLC and other neuroendocrine cancers, a tissue sample is required. Evaluation and/or diagnosis is based upon light microscopy4,20-22
- Depending on tumor location and size, a biopsy may be obtained9
- The World Health Organization recognizes only 2 subtypes of SCLC: SCLC and combined SCLC3
- Combined SCLC has an additional component of non-small cell carcinoma, which can be of any non-small cell histological subtype3
HISTOPATHOLOGY OF SCLC TUMORS
- Histopathological diagnostic criteria include tumor cells of small size, a round-to-fusiform shape, scant cytoplasm, finely granular nuclear chromatin, and absent or inconspicuous nucleoli4,22
Pathology of a Tumor23
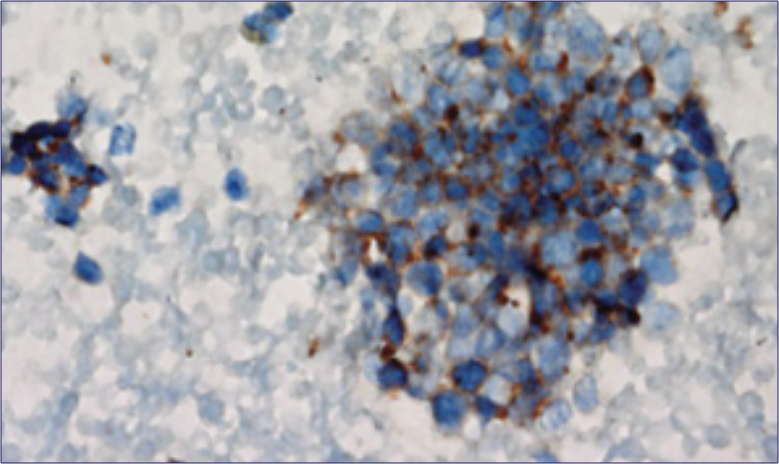
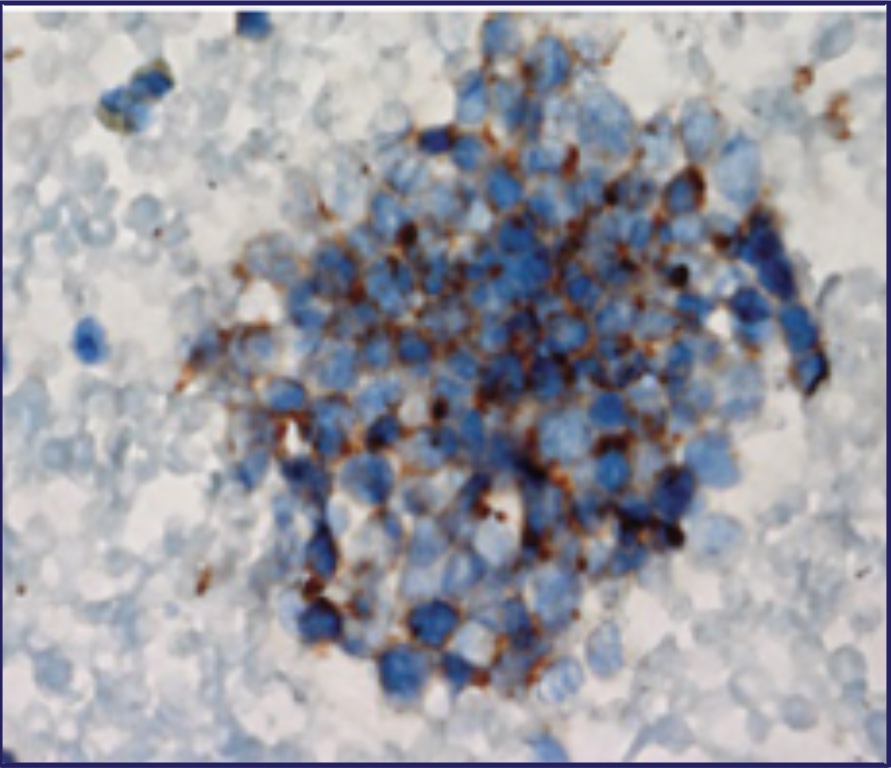
Reproduced, with permission, from Carter BW, Glisson BS, Truong MT, Erasmus JJ. Small cell lung carcinoma: staging, imaging, and treatment considerations. RadioGraphics. 2014;34(6):1707-1721.
There are standard immunohistochemical markers for lung origin and neuroendocrine differentiation that are useful for distinguishing SCLC from other tumor types.4
About Neuroendocrine Tumors
- SCLC is a poorly differentiated, high-grade neuroendocrine tumor, characterized by its aggressive spread throughout the body3,24
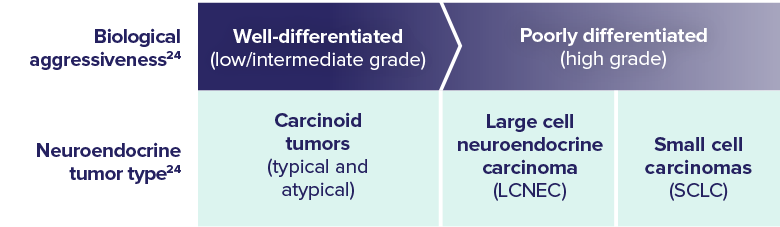
KEY PATHOLOGY FEATURES
- SCLC cells frequently exhibit expression of genetic abnormalities and other molecular characteristics implicated in driving oncogenesis25
Select Molecular Characteristics of SCLC
-
Driver mutations include:
- P53 and RB mutations, detected in up to 90% of SCLC tumors
- In contrast to NSCLC, mutations in the EGFR and KRAS oncogenes are rare, except in occasional cases of SCLC transformation of EGFR-mutated lung adenocarcinomas
-
There are no established biomarkers for SCLC.
- Clinical trials are ongoing
PDL-1 expression is associated with longer survival in SCLC patients. However, it is uncertain if PDL-1 expression is a determinant predictive biomarker.
-
Amplification of MYC promotes tumor progression by deregulating oncogenic transcription, cell cycle control, and metabolism.
- Genomic amplifications in MYC are observed in 6%-24% of patient tumors, and are more prevalent (32%-44%) in SCLC cell lines
Increased ASCL1, NEUROD1, and POU2F3 may regulate differentiation of pulmonary neuroendocrine cells which may lead to malignancy.
Misregulation of multiple transcription factors leads to abnormal neuroendocrine development, leading to tumor growth.
ASCL1=achaete-scute-like 1; EGFR=epidermal growth factor receptor; KRAS=Kirsten rat
sarcoma virus;
NEUROD1=neurogenic differentiation 1; P53=tumor protein 53; POU2F3=POU Class 2 Homeobox 3; RB=retinoblastoma.
- Gaps in the characterization of SCLC still remain, and clinical progress lags behind other disease states in discovering targetable pathways3
These select molecular characteristics may contribute to SCLC pathogenesis, and as such, could form the basis of pharmacologic treatment approaches.25,26
NCCN=National Comprehensive Cancer Network® (NCCN®)
-
REFERENCES:
- Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21(10):2244-2255.
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369(10):920-931.
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. doi:10.1038/s41572-020-00235-0
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Small Cell Lung Cancer V.3.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed December 21, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664-672.
- Huber RM, Tufman A. Update on small cell lung cancer management. Breathe. 2012;8(4):314-330.
- Small cell lung cancer treatment (PDQ®)–health professional version. National Cancer Institute. https://www.cancer.gov/types/lung/hp/small-cell-lung-treatment-pdq. Accessed May 7, 2021.
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
- Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94(8):1599-1622.
- Ferretti GR, Jankowski A. Lung cancer. In: Coche E, Ghaye B, de Mey J, Duyck P, eds. Comparative Interpretation of CT and Standard Radiography of the Chest. Medical Radiology. Springer, Berlin, Heidelberg; 2011:409-435.
- Danese M, Gleeson M, Lubeck D, Bobiak S. Total cost of care among patients with extensive disease small cell lung cancer. Poster presented at: the International Society for Pharmacoeconomics and Outcomes Research 22nd Annual International Meeting; May 20-24, 2017; Boston, MA. PHS65.
- Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e400S-e419S.
- Rintoul RC, Tournoy KG, El Daly H, et al. EBUS-TBNA for the clarification of PET positive intra-thoracic lymph nodes–an international multi-centre experience. J Thorac Oncol. 2009;4(1):44-48.
- Higdon ML, Atkinson CJ, Lawrence KV. Oncologic emergencies: recognition and initial management. Am Fam Physician. 2018;97(11):741-748.
- Soomro Z, Youssef M, Yust-Katz S, Jalali A, Patel AJ, Mandel J. Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis. 2020;12(10):6253-6263.
- Mayer S, Cypess AM, Kocher ON, et al. Uncommon presentations of some common malignancies: Case 1. Sequential paraneoplastic endocrine syndromes in small-cell lung cancer. J Clin Oncol. 2005;23(6):1312-1314.
- Gandhi L, Johnson BE. Paraneoplastic syndromes associated with small cell lung cancer. J Natl Compr Canc Netw. 2006;4(6):631-638.
- Titulaer MJ, Maddison P, Sont JK, et al. Clinical Dutch-English Lambert-Eaton Myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol. 2011;29(7):902-908.
- Ochenduszko S, Wilk B, Dabrowska J, et al. Paraneoplastic limbic encephalitis in a patient with extensive disease small-cell lung cancer. Mol Clin Oncol. 2017;6(4):575-578.
- Dorantes-Heredia R, Ruiz-Morales JM, Cano-García F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5(4):401-412.
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26(9):1184-1197.
- Basumallik N, Agarwal M. Small cell lung cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021 Jan. https://www.ncbi.nlm.nih.gov/books/NBK430685/. Accessed May 7, 2021.
- Carter BW, Glisson BS, Truong MT, Erasmus JJ. Small cell lung carcinoma: staging, imaging, and treatment considerations. RadioGraphics. 2014;34(6):1707-1721.
- Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers. 2012;4(3):777-798.
- Christensen CL, Kwiatkowski N, Abraham BJ, et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014;26(6):909-922.
- Kim D-W, Kim K-C, Kim K-B, Dunn CT, Park K-S. Transcriptional deregulation underlying the pathogenesis of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):4-20.
- Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165-e172.
- Tsoukalas N, Aravantinou-Fatorou E, Baxevanos P, et al. Advanced small cell lung cancer (SCLC): new challenges and new expectations. Ann Transl Med. 2018;6(8):145. doi:10.21037/atm.2018.03.31
- Mollaoglu G, Guthrie MR, Böhm S, et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell. 2017;31(2):270-285.
- Huang Y-H, Klingbeil O, He X-Y, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32(13-14):915-928.
